The Metabolic Miracle: A Petroleum Bioremediation Refresher
By: Cascade EnvironmentalBioremediation is a crucial component of many remediation plans. In fact, experts forecast the global bioremediation market will reach $186.3 billion by 2023. Here in the U.S., petroleum bioremediation is among the most common in situ treatment remedies for sites contaminated with organic compounds. Understanding how it works can help you to make more informed decisions about its applications and performance. In this blog post, we’ll provide a refresher on the miracle of bioremediation.
WHAT IS BIOREMEDIATION?
Every day, a new article is published about the importance of bacteria to the larger ecosystem. Our personal bacterial microcosm, specifically the bacteria in our gut, plays a major role in regulating a wide variety of biological processes. It’s a symbiotic relationship, and it’s similar to what we seek to establish in the subsurface with bioremediation.
To create that mutually beneficial condition, we intervene to influence the conditions in the subsurface by stimulating microorganisms with nutrients and other chemicals to enable them to destroy contaminants. There are three things we need to consider to do this successfully:
- The type of contaminant in the subsurface
- The subsurface geological and chemical conditions
- The type of microorganisms that will be utilized or stimulated
For petroleum contaminants, we can adjust the environmental conditions and/or introduce the right microorganisms. Existing conditions can be enhanced by adding oxygen and nutrients to produce aerobic degradation, or by adding sulfates/nitrates for anerobic degradation, depending on the oxidation reduction potential (ORP) and dissolved oxygen content of groundwater. Injecting nutrients or oxygen does not destroy contaminants directly, but creates an environment that encourages the growth of a healthy population of microorganisms that do the degrading for us.
HOW DOES BIOREMEDIATION WORK?
Bioremediation occurs when microorganisms gain energy by catalyzing reactions that deteriorate chemical bonds, then transfer electrons away from the contaminant in an oxidation-reduction reaction. In simpler terms, bacteria gain energy from the process of breaking down hydrocarbon contaminants in the presence of oxygen. The hydrocarbon is broken down into carbon that is used to multiply and electrons that are used for energy to power the cell.
AEROBIC DEGRADATION
Petroleum hydrocarbons are a string of carbon and hydrogen atoms in a stable molecule. The petroleum hydrocarbon contaminants we see most often in our industry are fuel oils such as gasoline and diesel, or their main chemical components: benzene, toluene, ethylbenzene, and xylenes (BTEX). The process of destroying organic compounds with the aid of oxygen is called aerobic respiration, and interestingly, the byproducts are the same byproducts of the human respiration process: carbon dioxide and water. Another byproduct is a healthy community of microorganisms.
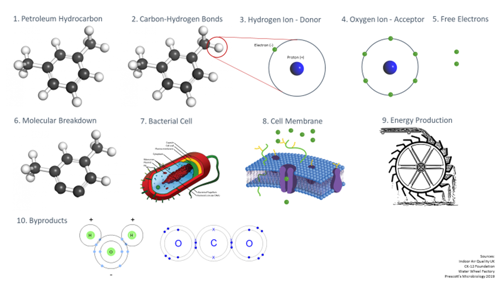
Let’s take a closer look at the aerobic respiration process, step by step.
- We start with a petroleum hydrocarbon contaminant in the subsurface environment.
- The molecular composition of the hydrocarbon is a series of carbon-hydrogen bonds – carbon in black and hydrogen in white.
- Zooming in on the hydrogen atom here, in its unbonded form, we see it’s composed of a single proton in the nucleus and single electron surrounding it.
- Also present in this environment are oxygen ions and, as we may remember from high school chemistry, oxygen atoms have six electrons in their outer shell (or valence shell), but this is unstable, as atoms like to have eight electrons for a balanced, symmetrical atomic structure.
- To reach that stable eight electron structure, the oxygen ion’s stronger pull will rip away one electron from each of two hydrogen atoms. The contaminant is now oxidized—it has lost electrons, which is why it is called the electron donor. Oxygen gained electrons and is called the electron acceptor.
- Now we see two hydrogen atoms removed from the hydrocarbon chain, and we can see how repeated attacks by the oxygen molecule will start to break this molecule down.
- Those electrons don’t always instantaneously bond with the oxygen, as there are lots of things happening in the environment: bacteria are also present here, and they have a use for those electrons.
- Zooming in on the membrane of the cell, we have the phospholipid bilayer, which is a polar membrane of fat cells with various embedded signal acceptors and ion pumps. Outside that membrane, we see a group of electrons creating a net negative charge, while inside the membrane we see fewer electrons. We know the natural tendency of an environment is to move towards a balance from charged to neutral whenever there is a concentration gradient
- In the process of obtaining even distribution through diffusion across the membrane, the cell uses that motion to generate energy. It’s similar to the way water flows due to gravity (analogous to the concentration gradient) from higher to lower elevation, and how the natural action of water flow can be harnessed to do work, like a water wheel turning to do the work of driving a mill to grind flour.
- The end products, after all of the subsurface processes are complete, are the same as what started, but rearranged—the hydrogen is now combined with oxygen, and carbons combined with oxygen thanks to oxygenase enzyme activators. We’ve produced carbon dioxide and water.
ANAEROBIC DEGRADATION
The second type of bioremediation is anaerobic degradation and it’s more complicated, but here we’ll provide a general overview.
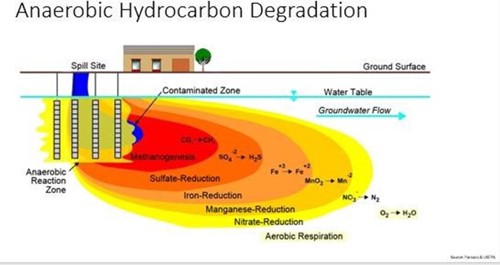
Image credit: CLU-IN.org
In the subsurface environment, there is more than just oxygen and bacteria. When the oxygen in the system is used up and the environment is anaerobic, other electron acceptors step in to provide energy for the bacteria. This occurs in a stepwise fashion, following the natural law of path of least resistance, easiest to most difficult, to free up electrons—or those of higher oxidation potential first, then lower oxidation potential. For example, in nitrate reduction the microbe is able to break nitrate (NO3) to provide an oxygen atom and convert the molecule to nitrite (NO2). Later in the process, semi-unstable metals are used in another complex process to free up an oxygen atom that will degrade the hydrocarbon and continue to advance the metabolic process.
This figure is a little misleading because it makes it seem like this process goes from near-source to downgradient, but actually it’s the opposite. The image depicts a late-stage spill, and the source area was where aerobic biodegradation occurred until biological oxygen demand was higher than the dissolved oxygen available—then nitrate, then so on until CO2 is the only thing left and creates a much slower breakdown process.
These anerobic reactions produce different byproducts than aerobic respiration: nitrate reduction produces nitrogen gas, sulfate reduction produces hydrogen sulfide (H2S), and conversion of CO2 produces methane (CH4).
WHAT SUBSTANCES ARE INJECTED FOR BIOREMEDIATION?
As explained earlier, the amendments that are chosen for each site depend on the contaminant and the geological and chemical conditions. However, there are some common substances injected during bioremediation:
- Injectable amendments that act to create either aerobic or anaerobic conditions in the treatment zone:
- Direct addition of oxygen for petroleum hydrocarbons, typically in the form of a solid slow release calcium peroxide;
- Insoluble emulsified vegetable oil (EVO) and soluble sodium lactate generate anaerobic conditions for chlorinated solvents.
- Organic and inorganic nutrients to support microbial population growth:
- Yeast extract;
- Nitrogen;
- Phosphorous.
- Buffers to modify pH to optimal range friendly to the existing or introduced bacteria
- Vitamins to support healthy function
HOW DO WE KNOW IF BIOREMEDIATION IS WORKING?
We use a variety of field and analytical indicators, such as…
- Field-Measured Parameters
- High total dissolved solids (TDS) – as evidenced by cloudy water wherever the amendment has reached monitoring wells or other groundwater sampling points;
- Dissolved oxygen content rising or falling, depending on which aerobic or anaerobic approach you’re using;
- pH changes if buffers are used, to keep pH in optimized conditions for the bacteria to thrive;
- – increasing or decreasing trend indicates tendency to donate or accept electrons.
- Contaminant concentrations changing over time
- Molecular biology/genomic testing – baseline compared to post-injection bacterial population sampling
- Terminal electron acceptors (TEAs) – concentrations over time
- Sulfate/Sulfite;
- Nitrate/Nitrite;
- Dissolved ferrous/ferric iron;
- Dissolved manganese with speciation, if possible.
- Byproducts
- Dissolved CO2
- Geochemical indicators
- Total phosphorous
WHAT ARE OTHER BIOREMEDIATION APPROACHES THAT CAN BE USED?
Although the methods described in this blog post are the most common, there are many bioremediation approaches available:
- Monitored natural attenuation (MNA) – The passive approach, which allows the natural bioremediation processes to occur under our supervision and tracking. This is also referred to as intrinsic bioremediation.
- Cometabolism - A variation on biodegradation in which microbes destroy a contaminant even though the contaminant cannot serve as the primary energy source for the organisms. It’s an incidental reaction catalyzed by enzymes involved in cell metabolism. To degrade the contaminant, the microbes require the presence of other compounds that can support their growth. For example, when bacteria oxidize methane, they produce enzymes that incidentally degrade chlorinated solvents.
- Fermentation – A type of metabolism in anaerobic environments where the contaminant serves as both electron donor and electron acceptor in a series of internal electron transfers catalyzed by the microorganism. It produces ethanol, hydrogen, and CO2, which in turn are degraded further by other species of bacteria.
Bioremediation is a fascinating example of how we can team up with nature to remove contaminants ranging from chlorinated solvents to petroleum hydrocarbons.
If you’d like more information about how to use bioremediation on an upcoming project, you can check out a case study, reach out to me or request a quote.